
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
Active Pharmaceutical Ingredient (API)
BP / USP (as applicable)
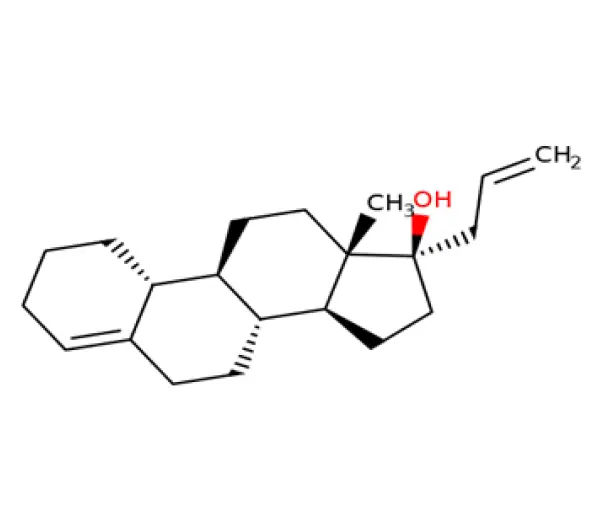
C₂₁H₃₂O₂
(17α)-17-(allyloxy)estr-4-en-3-one
432-60-0
316.48 g/mol
Synthetic Progestogen (Steroidal)
Prevention of threatened miscarriage, treatment of recurrent pregnancy loss
| Appearance | White to pale yellow crystalline solid. |
|---|---|
| Solubility | Slightly soluble in chloroform and methanol |
| Melting Point | 79.5-80 °C |
| pH | - |
Allyestrenol is a synthetic progestogen used mainly in pregnancy support. It helps maintain appropriate progesterone activity required for fetal development and pregnancy sustainability. It is widely used in obstetrics and gynecology for preventing miscarriage, managing threatened abortion, habitual miscarriage, and certain pre-term labor risks. With good global demand, particularly in developing markets, Allyestrenol is supplied as a high-purity API for tablet formulations.
Allyestrenol is a synthetic progestogen that mimics the action of natural progesterone. It binds to progesterone receptors in the uterus, helping maintain a supportive environment for pregnancy. By stabilizing the endometrium, it reduces uterine contractions and prevents premature labor.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Allyestrenol is generally used during early to mid-pregnancy. Continued use throughout the entire pregnancy should only be under strict medical supervision.
Allyestrenol may interact with drugs that affect liver metabolism, including certain anticonvulsants or antibiotics. Always inform your doctor about all medications you are taking.
Long-term use should be monitored by a physician. Regular check-ups may be needed to assess liver function, hormone levels, and overall maternal health.
Common side effects may include nausea, headache, dizziness, breast tenderness, or mild fatigue. Serious side effects are rare.
No. Always consult your doctor before stopping or changing the dose. Stopping abruptly may affect your treatment.
Looking to source Allyestrenol or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.